There are millions of vehicles on roads today.
Majority of them are run by
Internal combustion engines become a major contributor to air pollution. Especially in cities,
Where huge traffic is unavoidable can result in poor air
Quality that can affect not just the environment, but even our health.
To solve this problem,
The governments of different countries around the globe;
Have taken the necessary steps to implement stricter emission norms for vehicles.
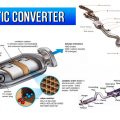
Catalytic converter is one of the devices,
That could lessen the intensity of harmful emissions coming out of a vehicle.
What is a Catalytic Converter?
Catalytic converter is a device that converts
Harmful toxic emission gases from a vehicle into less harmful emission gases.
The process is achieved either through oxidation or reduction.
Catalytic converters can be used to treat exhaust gases of;
Both gasoline and diesel engines.
History of Catalytic Converters:
Eugene Houdry,
A French Mechanical Engineer invented the catalytic converter.
He was awarded a patent by the U.S. Government for his research on developing,
Catalytic converters for a gasoline engine.
Catalytic converters were a failure then due to the presence of tetraethyl lead in gasoline. Presence of lead in gasoline poisoned the catalytic converters and disabled,
It from treating exhaust gases. U.S. Government started phasing out the use of
Leaded gasoline in the mid-1970s because of its neurotoxicity and;
Its degrading effect on catalytic converters.
In 1996, U.S.A completely banned the use of leaded gasoline.
The first catalytic converter for production was developed by engineers such a John J. Mooney and Carl D. Keith at the Engelhard Corporation.
3-way Catalytic Converters
A 3-way catalytic converter is used to treat the;
3 main harmful gases coming out of exhaust such as the nitrogen oxides (NOX),
Hydrocarbons (HC) and carbon monoxide (CO) emissions.
There are 2 different catalysts at work: one is the reduction catalyst,
And the other is the oxidation catalyst. The reduction catalyst is made of platinum and rhodium. The oxidation catalyst is made of platinum and palladium.
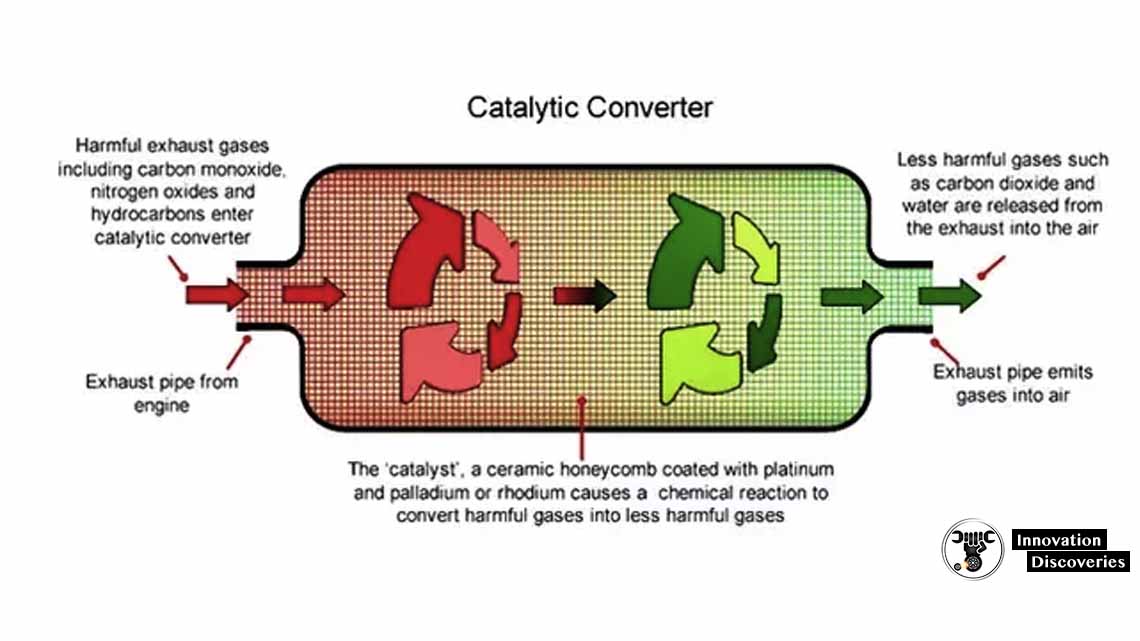
Alumina Coating:
The catalysts are in the form of thousands of micro ducts that resemble the form of a honeycomb. The gases pass through these catalyst ducts. The honeycomb structure is applied with a coat of alumina (aluminum oxide). Alumina is highly porous which greatly
Increases the surface area of the structure. The alumina coat carries the precious catalyst materials which help in oxidation and reduction. This design maximizes the surface area of the catalyst,
Thus, giving it more gases to react on.
Catalytic converter is installed between exhaust pipe and muffler. It works best when it is heated and can give efficiency up to 90%. As a result, we get less harmful toxic gases from the tailpipe.
Working of a catalytic converter:
The exhaust gases from the engine are first sent through the reduction catalyst.
The reduction catalyst (platinum + rhodium)
Will try to eliminate NOX as much as possible.
Reaction at reduction catalyst:
When NOX (NO or NO2) molecules pass through the contact area,
The catalyst breaks the bond between nitrogen and oxygen atoms.
Since it is a reduction reaction;
The catalyst will try to get rid of oxygen atoms and would attract the nitrogen atoms. The individual oxygen atoms (O) pair up with other
Oxygen atoms (O) to form an oxygen molecule (O2).
The nitrogen atoms (N) stick to the catalyst surface unless they find other nitrogen atoms (N) to bond with to form nitrogen molecules (N2).
2 NO → N2 + O2
(or)
2 NO2→ N2 + 2O2
The reduction catalyst won’t eliminate the CO and HC molecules.
Also, read – Exhaust Gas Recirculation (EGR)
Reaction at oxidation catalyst:
The oxidation catalyst is another honeycomb-shaped structure made of platinum and palladium. Since it is an oxidation reaction; the catalyst will try to attract the oxygen atoms. The CO bond is strong enough to be split by the catalyst. Thus, the CO molecule as a whole will be attracted to the catalyst surface. The catalyst surface will also attract oxygen molecules (O2).
Now, since O2 molecules’ bonds are weaker than the CO bond,
The O2 is split into individual oxygen atoms (O). The oxygen atoms will bond with the CO molecules to form CO2.
2CO + O2 → 2CO2
The hydrocarbon (HC) molecules also get treated by the oxidation catalyst. When HC molecules come in contact with the catalyst surface,
They are split into hydrogen (H) and carbon (C). Both hydrogen and carbon bond with
Oxygen to form water vapor (H2O) and carbon dioxide (CO2).
C8H18 + 17O2 → 8CO2 + 18H2O
(or)
CH4 + 2O2 → CO2 + 2H2O
Etc….
Also, read – Exhaust Gas Recirculation (EGR)
See More: