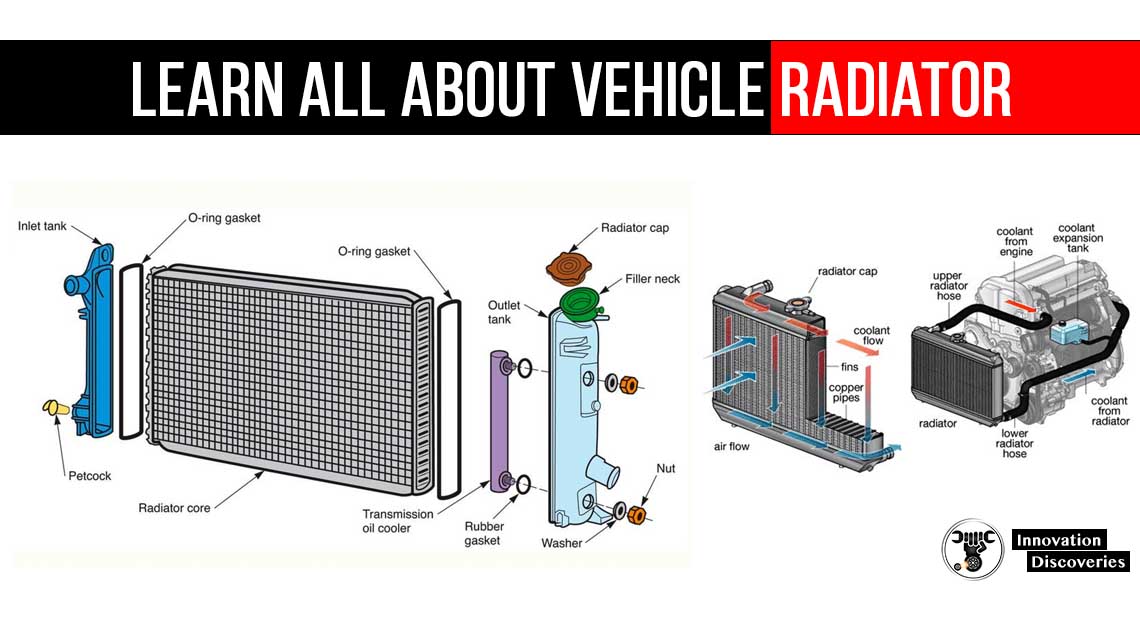
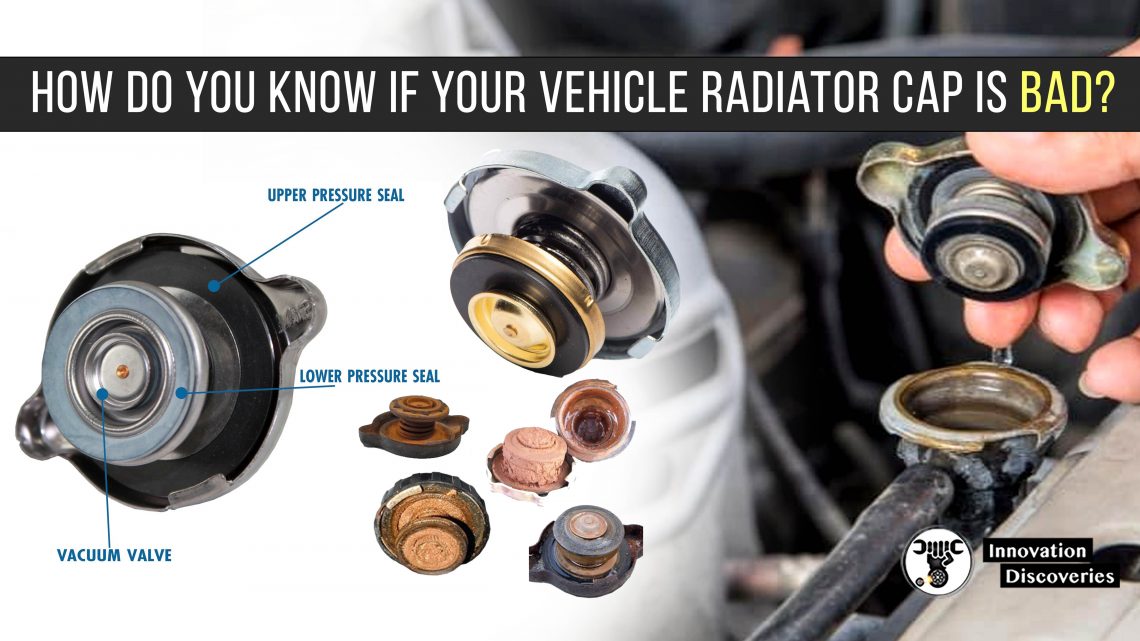
Automotive cooling systems are designed to maintain an engine’s temperature within an optimal range—usually between 90°C and 105°C. If the coolant freezes in cold conditions or boils under heavy load, the consequences can be catastrophic: cracked blocks, warped cylinder heads, blown gaskets, or total engine seizure. This is where coolant additives play a critical role, specifically those engineered to raise the boiling point and lower the freezing point of the coolant.
🔬 The Thermodynamics of Coolant
At its core, coolant is a mixture of water and ethylene glycol (or propylene glycol). Water alone has excellent heat transfer capability but freezes at 0°C and boils at 100°C under standard atmospheric pressure. For modern engines, these limits are insufficient.
By adding glycol-based antifreeze agents, we alter the colligative properties of the solution:
- Freezing Point Depression: The presence of solute molecules interferes with the formation of ice crystals, meaning the liquid remains fluid even below 0°C.
- Boiling Point Elevation: The solution requires more energy to vaporize, effectively raising its boiling threshold.
This principle is rooted in Raoult’s Law, which governs how solutes influence vapor pressure and phase transitions.

⚗️ Boiling Point Additives Explained
Coolant additives like ethylene glycol can raise the boiling point of water significantly. For example:
- Pure water boils at 100°C at 1 atm.
- A 50/50 water-glycol mix can raise this boiling point to 106–110°C.
- With a pressurized radiator cap (typically 15 psi), the effective boiling point climbs further to 125–130°C.
This ensures that under heavy loads (e.g., towing, high-speed driving, desert climates), coolant remains in liquid form, maintaining efficient thermal transfer and preventing vapor lock.

❄️ Freezing Point Additives Explained
In sub-zero conditions, water expands upon freezing, potentially cracking engine blocks. Glycol additives prevent this by lowering the freezing point of the coolant mixture.
- A 50/50 mix of water and ethylene glycol protects down to about –37°C.
- A 70/30 mix extends protection to nearly –55°C, though this reduces heat transfer efficiency due to the higher glycol concentration.
Thus, the mixture ratio must balance thermal efficiency and freeze protection.

🛡️ Secondary Benefits of Additives
Beyond temperature control, additives are engineered with corrosion inhibitors, pH stabilizers, and anti-foaming agents. These protect aluminum radiators, water pumps, cylinder heads, and gaskets from electrochemical degradation and cavitation damage.
Types of inhibitors include:
- IAT (Inorganic Additive Technology): Silicate & phosphate-based, common in older vehicles.
- OAT (Organic Acid Technology): Longer-lasting, typically 5 years or 150,000 km.
- HOAT (Hybrid Organic Acid Technology): A blend for modern engines.

🚗 Practical Engineering Considerations
- Mixture ratio: Always maintain the recommended ratio (commonly 50/50). Too much water reduces protection; too much glycol reduces heat dissipation.
- System pressure: Radiator caps are calibrated to raise pressure, which directly increases the boiling point.
- Coolant type compatibility: Mixing different technologies (IAT, OAT, HOAT) can lead to precipitation and clogging.

⚙️ The Chemistry of Coolant Molecules
Most automotive coolants use glycol-based antifreeze agents, commonly ethylene glycol (EG) or propylene glycol (PG):
🔹 Ethylene Glycol (C₂H₆O₂) – Formula: HOCH2CH2OHHOCH₂CH₂OHHOCH2CH2OH
- Excellent heat transfer properties
- Freezing point: –12.9 °C (pure)
- Toxic if ingested
🔹 Propylene Glycol (C₃H₈O₂) – Formula: CH3CH(OH)CH2OHCH₃CH(OH)CH₂OHCH3CH(OH)CH2OH
- Safer, lower toxicity (used in food-related industries too)
- Slightly less efficient in heat transfer than EG
🧬 How They Work:
Water freezes at 0 °C, but adding glycol molecules disrupts hydrogen bonding in water. This prevents ice crystal formation → lower freezing point. Similarly, glycol raises water’s boiling point → coolant can handle hotter engine temps before vaporizing.
🌡️ Boiling & Freezing Point Elevation Explained
- Freezing Point Depression – Glycol interferes with water’s crystal lattice, lowering the freezing point (up to –37 °C for a 50:50 mix).
- Boiling Point Elevation – Glycol molecules increase liquid stability, raising the boiling point (≈130 °C in a pressurized cooling system).
- Pressure Effect – Radiator caps maintain 1.0–1.5 bar pressure → further elevating boiling point by 20–25 °C.
📊 Boiling Point Curve Science
Coolant performance can be explained using thermodynamic diagrams:
1. Heating Curve (Pure Substances)
- Y-axis: Temperature, X-axis: Heat input
- Flat plateau = boiling phase change
- Example: Water holds at 100 °C until all liquid becomes vapor
2. Phase Diagram (Pressure vs Temperature)
- Shows liquid-vapor equilibrium curve
- At higher pressure → higher boiling point
- Includes triple point (solid, liquid, vapor coexist) and critical point (supercritical fluid beyond which liquid/gas distinction vanishes).
3. Boiling-Point Composition Curve (Mixtures)
- Used for water + glycol systems
- Two curves: liquid composition vs vapor composition
- Explains why a 50:50 mix balances freezing protection, boiling resistance, and pumpability.
🛠️ Additives Beyond Glycol
A complete coolant is more than water + antifreeze. It includes:
- Corrosion inhibitors → prevent rust on aluminum, iron, and copper
- pH stabilizers → stop acid buildup
- Dyes → green, orange, blue for leak detection
- Anti-foaming agents → maintain fluid consistency under pump cavitation
🔥 Why It Matters
- Prevents engine block cracking in extreme cold ❄️
- Stops overheating in summer traffic jams 🔥
- Protects metal surfaces from corrosion ⚙️
- Extends engine life and efficiency 🚗💨
❓ FAQ – Coolant Boiling & Freezing Point Additives
1️⃣ Can I just use water instead of coolant?
No. While water has excellent heat transfer properties, it freezes at 0°C and boils at 100°C, which is unsafe for modern engines. Coolant additives prevent freezing, boiling, corrosion, and cavitation damage.
2️⃣ What is the ideal water-to-coolant ratio?
A 50/50 mix is standard, balancing freeze protection (–37°C), boiling point elevation (~130°C with pressure), and optimal heat transfer. Some extreme climates may require slight adjustments (e.g., 60/40).
3️⃣ Can I mix different types of coolants (IAT, OAT, HOAT)?
Mixing coolant types is not recommended. Different additive chemistries can react, forming precipitates that clog radiators, pumps, and hoses. Always check manufacturer recommendations.
4️⃣ How does coolant prevent corrosion?
Coolants contain corrosion inhibitors and pH stabilizers that protect aluminum, copper, and iron parts from electrochemical damage, rust, and pitting over time.
5️⃣ How often should I replace coolant?
Depends on the coolant type:
- IAT (Inorganic Additive Technology): ~2 years or 40,000 km
- OAT (Organic Acid Technology): ~5 years or 150,000 km
- HOAT (Hybrid OAT): Varies by manufacturer; usually 4–5 years
6️⃣ What happens if the coolant freezes or boils?
- Freezing: Can crack engine blocks or damage radiator components.
- Boiling: Leads to vapor lock, loss of heat transfer, and potential engine overheating.
7️⃣ Can coolant improve engine performance?
Indirectly, yes. By maintaining stable engine temperatures, coolant ensures the engine runs efficiently, reduces wear, and prevents thermal stress that could lower performance.
✅ Conclusion
The science behind coolant boiling point and freezing point additives is rooted in thermodynamics and materials chemistry. These additives not only ensure an engine runs safely across extreme climates but also extend component life and preserve performance.
Understanding the role of colligative properties and chemical inhibitors helps us appreciate why choosing the correct coolant formulation is vital for engine health.
Discover More:


Read More:
- WORKING OF THERMOSTATS
- CAR THERMOSTAT FUNCTIONS, FAILURE SYMPTOMS, AND REPLACEMENT COST
- PISTON DAMAGE FROM OVERHEATING
- SYMPTOMS OF AN EXHAUST LEAK
- EXHAUST GAS RECIRCULATION (EGR)
- HOW AN ENGINE COOLING SYSTEM WORKS
- WATER COOLING VS AIR COOLING
Read: BLEED A RADIATOR AT HOME EASILY
HOW TO APPLY A TEMPORARY REPAIR FOR UPPER RADIATOR HOSE?
Visit Forum
Visit Our Friendly Website